Release time :2025-02-14
Source:support@yingchitech.com
Scan:635
In January 2025, Professor Duan Xujun's team from the University of Electronic Science and Technology published a groundbreaking study in the internationally renowned journal Biological Psychiatry, titled "Personalized theta-burst stimulation enhances social skills in young minimally verbal children with autism: a double-blind randomized controlled trial". The study utilized personalized brain imaging analysis technology and implemented a continuous theta burst stimulation (cTBS) protocol to precisely target the amygdala and its neural circuits in children with autism. This intervention achieved significant therapeutic effects.

This study, conducted as a double-blind randomized controlled trial, investigated the effects of personalized continuous theta burst stimulation (cTBS) on improving social skills in children with autism who have language impairments. A total of 44 children with autism aged 2–8 years were enrolled and divided into two groups: a personalized stimulation group optimized for amygdala functional connectivity (amygdala-optimized functional connectivity, AOFC) and a non-optimized (NO) control group with standard prefrontal stimulation. The results showed that the AOFC group demonstrated significant improvements in social and communication scores on the Autism Diagnostic Observation Schedule (ADOS). Additionally, amygdala volume, spontaneous activity, and functional connectivity exhibited significant changes, which correlated with clinical improvements. The study demonstrated that personalized cTBS can improve core autism symptoms by modulating the structure and functional connectivity of the amygdala, providing important evidence for the development of precise therapeutic strategies for this special population.
Autism Spectrum Disorder (ASD) is a lifelong neurodevelopmental disorder that typically manifests in early childhood. It is considered one of the major global public health challenges. According to data from the China Disabled Persons' Federation, there are approximately 13 million individuals with autism in China, including around 1 million children aged 2 to 8 years. The incidence rate among newborns exceeds 1%. The exact causes of ASD remain unknown, and its pathological mechanisms, both cross-sectional and longitudinal, are highly complex. Clinical phenotypes are strongly heterogeneous, and effective diagnostic and therapeutic approaches are lacking, with generally poor prognoses. Additionally, preventive measures are severely limited. The high prevalence and severe impact of ASD underscore the urgent need for early identification and intervention.
Early intervention is crucial for improving ASD symptoms, and transcranial magnetic stimulation (TMS) has emerged as a promising therapeutic approach for various psychiatric and neurological disorders. Continuous theta burst stimulation (cTBS), a variant of repetitive TMS, offers distinct advantages due to its shorter stimulation duration and longer-lasting aftereffects, making it particularly suitable for pediatric populations.
The amygdala has been identified as a viable therapeutic target for addressing social, communication, and emotional deficits in ASD. As a key component of the "social brain," the amygdala plays a pivotal role in processing social cues, recognizing emotions, evaluating social information, and regulating emotional responses. Its extensive connectivity with cortical regions, including the prefrontal cortex, underscores its broad influence on social and emotional behaviors. Structural, functional, and connectivity abnormalities in the amygdala are prominent in children with ASD and may directly contribute to social deficits.
The research team tested three key hypotheses regarding the fundamental mechanisms underlying autism interventions, which collectively form the theoretical basis for intervention strategies and explore the potential of non-invasive neuromodulation techniques in ASD treatment:
Non-invasive neuromodulation techniques can mimic the effects of invasive stimulation by targeting functional networks in cortical and subcortical brain regions, thereby modulating the structure and function of the amygdala associated with ASD.
The long-term depression (LTD) effects induced by cTBS may counteract the amygdala's overgrowth and hyperconnectivity observed in children with ASD, leading to improvements in social and behavioral deficits.
Improvements in social functioning in children with ASD are associated with changes in functional connectivity within amygdala-centered networks.
To validate these hypotheses, the research team designed a personalized cTBS strategy based on Amygdala-Optimized Functional Connectivity (AOFC) to target prefrontal cortical regions most strongly connected to the amygdala. Using a randomized, double-blind, controlled design, the study evaluated the effects of this AOFC-guided cTBS protocol in children with ASD who have limited language abilities.
The results demonstrated that the personalized AOFC-guided cTBS protocol had a significant effect in alleviating core ASD symptoms and revealed the neurobiological mechanisms underlying the treatment response. The findings highlighted the plasticity of the amygdala's structure, activity, and connectivity, which contributed to clinical improvements.
This research offers a promising pathway for developing targeted, personalized treatment strategies, particularly for children with ASD who have extremely limited language abilities.
A total of 58 children with autism (aged 2–8 years, clinically diagnosed with ASD according to DSM-5) were recruited for the study, with 8 excluded for not meeting ADOS criteria. Ultimately, 44 participants who completed baseline MRI scans were randomly assigned to the AOFC group (n = 23) and the NO group (n = 21).
In the AOFC group, all 23 participants completed the treatment, 22 completed post-treatment ADOS and behavioral scale assessments, and 18 completed post-treatment MRI scans. In the NO group, 19 participants completed the treatment, 17 completed post-treatment ADOS assessments, 15 completed behavioral scale assessments, and 15 completed post-treatment MRI scans.
The two groups were matched at baseline in terms of demographics and ADOS scores, except for the Repetitive and Restricted Behaviors (RRB) domain.

A double-blind randomized controlled trial (DB-RCT) was conducted, with participants randomly assigned to one of two groups: the AOFC group, which received personalized amygdala connectivity-guided cTBS, and the non-optimized (NO) control group, which received stimulation at the EEG F3 site. Randomization was performed by researchers not involved in the treatment. Baseline assessments included the Autism Diagnostic Observation Schedule (ADOS), parent-reported behavior scales, and MRI scans. Both groups underwent cTBS treatment over a 20-day period (4 weeks, 5 weekdays per week), with post-treatment assessments conducted within three days of the treatment's conclusion.
The cTBS protocol utilized a magnetic stimulator (M-100 Ultimate, YINGCHI Technology Co., Ltd., Shenzhen, China) and a figure-8 coil to deliver cTBS stimulation. Target stimulation sites were identified using a TMS navigation system (Quiks Vision, YINGCHI Technology Co., Ltd., Shenzhen, China) and marked on a swim cap. Each cTBS session consisted of bursts of three 50 Hz pulses repeated every 200 milliseconds, totaling 600 pulses per session. Three treatment sessions were conducted daily (1,800 pulses per day), with each session lasting 40.04 seconds and separated by 10-minute and 20-minute intervals. Stimulation intensity was set at 90% of the resting motor threshold.
Participants, clinical assessors, and treatment providers were blinded to the treatment assignments. Participants were randomly allocated to either the AOFC or NO group, using the same stimulation coil and target markings. The NO group underwent identical targeting and stimulation procedures as the AOFC group, preventing participants from distinguishing their group allocation. Caregivers of the participants were instructed not to discuss treatment assignments with staff or other participants to maintain the integrity of the double-blind design.
MRI scans were conducted using a 3T GE scanner with an 8-channel head coil. High-resolution T1-weighted images and resting-state functional MRI (fMRI) images were acquired. Each resting-state fMRI scan lasted 5 minutes and 10 seconds, consisting of 155 time points. To ensure data quality and participant comfort, MRI scans were performed under mild sedation.
Structural MRI analysis was processed using FreeSurfer software. Post-treatment changes in amygdala gray matter volume were evaluated using paired t-tests and Cohen's d effect sizes. Differences in the rate of volume change between the two groups were assessed with independent t-tests.
Resting-state fMRI analysis was performed using DPARSF software. Changes in the amplitude of low-frequency fluctuations (ALFF) in the amygdala during the resting state were analyzed using paired t-tests and Cohen's d. Functional connectivity changes between the amygdala seed region and the whole brain were assessed, and group comparisons were made for average connectivity changes. The functional connectivity strength (FCS) between the amygdala and intrinsic networks was also calculated.
Additionally, within-group analyses explored correlations between reductions in ADOS total scores and changes in FCS.
For the AOFC group, the personalized amygdala connectivity-guided target was generated based on baseline MRI scans. This process involved manual co-registration, fMRI preprocessing, individual T1 segmentation, and the definition of the amygdala and left dorsolateral prefrontal cortex (DLPFC) regions. The functional connectivity between the amygdala and the prefrontal cortex was then calculated to identify the voxels most strongly associated with the amygdala, which served as the personalized stimulation target.
The relationship between treatment response in the NO group and the location of prefrontal cortex stimulation was examined. Retrospective calculations were made for each participant's optimal stimulation target based on their pre-treatment amygdala functional connectivity maps. The functional connectivity strength between the F3 stimulation target and the amygdala was calculated, along with the Euclidean distance between the F3 stimulation target and the optimal target. These metrics were then correlated with the reduction in ADOS total scores.
The electric fields of the optimal stimulation target and the F3 target were modeled, and the correlation between the overlap of the electric field hotspots and the reduction in ADOS total scores was analyzed.
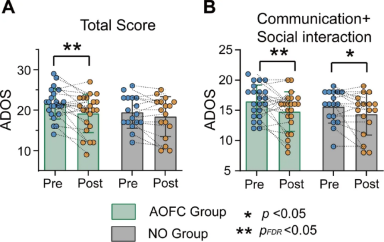
In the AOFC group, significant reductions were observed in both the total score and the communication and social interaction subscore, indicating that cTBS treatment effectively alleviated clinical symptoms of ASD. The statistical results are as follows:
Total score: t = 3.06, p = 0.005, pFDR = 0.029, Cohen’s d = 0.55
Communication and social interaction subscore: t = -2.51, p = 0.02, pFDR = 0.05, Cohen’s d = 0.56
In the NO group (non-optimized group), no significant changes were observed in either the total score or the communication and social interaction subscore, indicating no substantial improvement in this group. The specific statistical results are as follows:
Total score: t = -1.79, p = 0.09 (uncorrected), pFDR = 0.153, Cohen’s d = 0.24
Communication and social interaction subscore: t = -2.16, p = 0.046 (uncorrected), pFDR = 0.115, Cohen’s d = 0.40
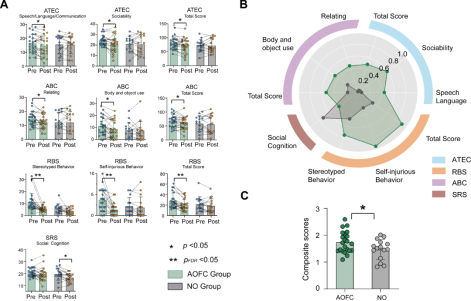
The team studied the effects of cTBS treatment on various ASD-related outcome measures: Autism Treatment Evaluation Checklist (ATEC), Autism Behavior Checklist (ABC), Social Responsiveness Scale (SRS), and Repetitive Behavior Scale (RBS). In the AOFC group, significant reductions were observed in the ATEC, ABC, and RBS total scores, while in the NO group, only the SRS score showed a significant decrease (Figure 2A). Effect sizes before and after treatment were compared, and in most symptom and behavior measures, the AOFC group consistently showed larger effect sizes than the NO group (Figure 2B). The average of all measurements from these scales was then calculated. The AOFC group demonstrated greater improvement in ASD-related outcome measures compared to the NO group (t = 2.09, p = 0.045, Cohen’s d = 0.70) (Figure 2C). These results indicate that after cTBS treatment, the AOFC group showed significant improvements across a range of ASD-related measures, with larger effect sizes observed in most measurements compared to the NO group, which only showed a significant decrease in the SRS score.
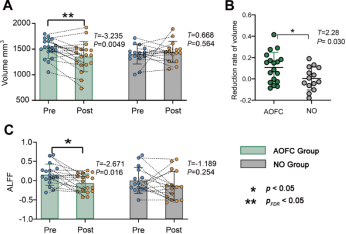
High-resolution MR data and brain structural segmentation based on FreeSurfer were used to examine changes in the amygdala’s gray matter volume. The AOFC group showed a significant decrease in right amygdala gray matter volume (t = -3.235, p = 0.0049, pFDR = 0.02, Cohen’s d = 0.64) (Figure 3A). The NO group did not show significant changes in right amygdala volume (t = 0.668, p = 0.564, Cohen’s d = 0.24). Between-group analysis revealed that the AOFC group had a significantly higher reduction in right amygdala gray matter volume (t = 2.28, p = 0.030, Cohen’s d = 0.81) (Figure 3B). No significant changes were observed in the left amygdala for either group. These results suggest that cTBS treatment led to a significant decrease in the right amygdala gray matter volume in the AOFC group, whereas no such change occurred in the NO group, highlighting the effect of personalized stimulation on amygdala structure.
Resting-state fMRI data measured the local functional changes in the amygdala following cTBS treatment. The AOFC group showed a significant decrease in ALFF in the right amygdala (t = -2.671, p = 0.016, Cohen’s d = 0.85) (Figure 3C). The NO group showed no changes in the right amygdala (t = -1.189, p = 0.254, Cohen’s d = 0.39). No significant changes were observed in the left amygdala for either group. These results suggest that cTBS treatment in the AOFC group led to a significant reduction in spontaneous neural activity in the right amygdala, whereas no such change was observed in the NO group.
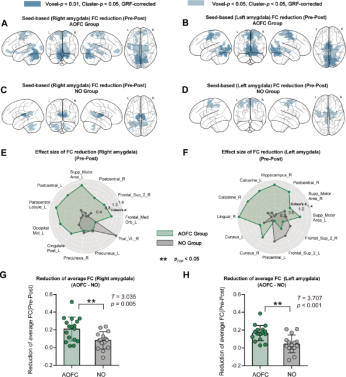
The study examined the overall changes in amygdala functional connectivity following cTBS treatment. In the AOFC group, cTBS significantly reduced the functional connectivity of the right amygdala with multiple brain regions (Figures 4A, E). In the NO group, cTBS only significantly reduced the functional connectivity of the right amygdala with the precuneus and thalamus (Figure 4C). The average reduction in right amygdala functional connectivity was significantly greater in the AOFC group compared to the NO group (t = 3.035, p = 0.005, pFDR = 0.005, Cohen’s d = 1.075) (Figure 4G). After cTBS treatment, similar reductions in functional connectivity were observed in the left amygdala (Figures 4B, D, F). The average reduction in left amygdala functional connectivity associated with cTBS treatment was significantly higher in the AOFC group compared to the NO group (t = 3.707, p < 0.001, pFDR = 0.001, Cohen’s d = 1.31, Figure 5H). These results suggest that in the AOFC group, cTBS induced a reduction in right amygdala functional connectivity, with a more pronounced change compared to the NO group, indicating extensive modulation of amygdala network connectivity following personalized treatment.
The study examined the effects of cTBS on the functional connectivity between the amygdala and six large-scale intrinsic networks: the Visual Network (VN), Somatomotor Network (SMN), Dorsal Attention Network (DAN), Ventral Attention Network (VAN), Frontoparietal Network (FPN), and Default Mode Network (DMN). In the AOFC group, cTBS significantly reduced the functional connectivity between the right amygdala and VN (t=2.813, pFDR=0.036), SMN (t=3.872, pFDR=0.006), and VAN (t=2.254, pFDR=0.046). It also significantly reduced the functional connectivity between the left amygdala and VAN (t=3.157, pFDR=0.036). In the NO group, there were no significant changes in the functional connectivity between either the right or left amygdala and any of the six networks.
Next, the study explored the relationship between changes in network-level amygdala functional connectivity and treatment outcomes. It found that, in the AOFC group, the changes in right amygdala functional connectivity induced by cTBS were significantly correlated with reductions in the ADOS total score and functional connectivity with the DAN (r=0.645, pFDR=0.036), FPN (r=0.564, pFDR=0.040), and DMN (r=0.596, pFDR=0.039). However, this relationship was not observed for the left amygdala after FDR correction. In the NO group, changes in amygdala functional connectivity were not related to reductions in the ADOS total score. These results suggest that, in the AOFC group, cTBS significantly reduced the functional connectivity between the right amygdala and key intrinsic networks, and these reductions in connectivity were significantly correlated with the decrease in the ADOS total score.
The study aimed to explore whether the effectiveness of cTBS treatment in the NO group was influenced by the functional connectivity between the F3 target and the amygdala. It was found that the functional connectivity between EEG F3 and the amygdala was significantly correlated with a reduction in the ADOS total score (r=0.55, p=0.03). These results suggest that, in the NO group, the clinical response to cTBS treatment was associated with the degree of functional connectivity between the standardized EEG F3 stimulation target and the amygdala, highlighting the relevance of amygdala connectivity in achieving symptom improvement, even in non-personalized treatment protocols.
The study also examined whether the overlap of the electric fields between the best and F3 stimulation targets affected symptom reduction in the NO group. It was found that, at all examined thresholds, the field overlap in the NO group was positively correlated with the reduction in the ADOS total score. These results suggest that in the NO group, a greater overlap of the electric fields between the standard F3 and personalized best stimulation targets was associated with a more significant reduction in the ADOS total score. Therefore, the precision of the electric field alignment with an individual's optimal target is a key factor in improving TMS efficacy for alleviating ASD symptoms.
This study is the first to explore the effectiveness of personalized, connectivity-guided stimulation therapy in improving speech disorders in children with Autism Spectrum Disorder (ASD). A continuous theta burst stimulation (cTBS) protocol based on amygdala functional connectivity (AOFC) was developed and validated through a randomized controlled trial, demonstrating its impact on behavior and clinical improvements in children. The results showed that AOFC-guided cTBS significantly improved core social communication deficits in ASD children and led to significant changes in the morphology, spontaneous neural activity, and large-scale network connectivity of the amygdala. In contrast, no similar effects were observed in the control group. These findings provide important evidence for developing effective, personalized treatment strategies for children with autism-related speech disorders and hold promises for addressing the significant unmet clinical needs in this population.
Future research should focus on further optimizing the AOFC-guided cTBS protocol and exploring its application potential in a broader ASD patient population. Additionally, long-term follow-up studies are needed to analyze the durability of treatment effects and the underlying mechanisms, which will help refine personalized treatment plans. Furthermore, this study provides new insights for the field of autism treatment, emphasizing the importance of precise stimulation targeting. It is expected to drive the development of more personalized treatment strategies based on brain functional connectivity, offering strong support for improving the quality of life for children with ASD and their families.

1. This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.
2. Reference:
https://www.sciencedirect.com/science/article/abs/pii/S0006322325000241