
YINGCHI Established
2012.6
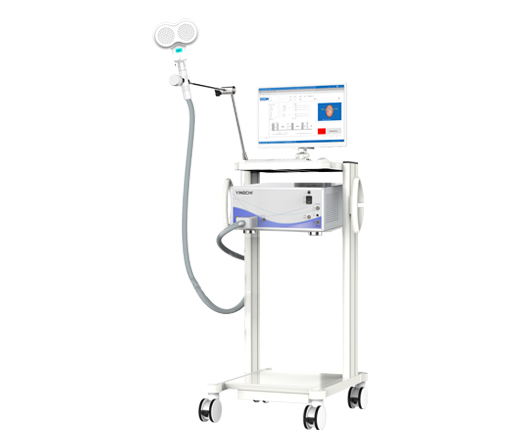
M-x Pro pulsed magnetic field stimulator obtained the medical device registration certificate Developed the S-x pulsed magnetic field stimulator prototype
2014.7

Obtained ISO 13485 system certification & ISO 9001 system certification Obtained CE certification by German TUV
2016.3
M-x Ultimate TMS is CE approved
2018.4
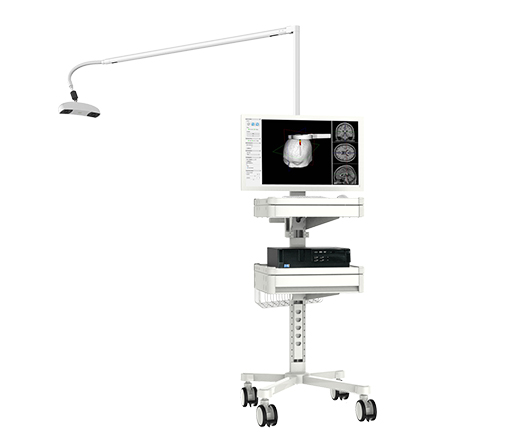
QuickVision Navigation Robotic Arm System has completed development and testing
2020.3
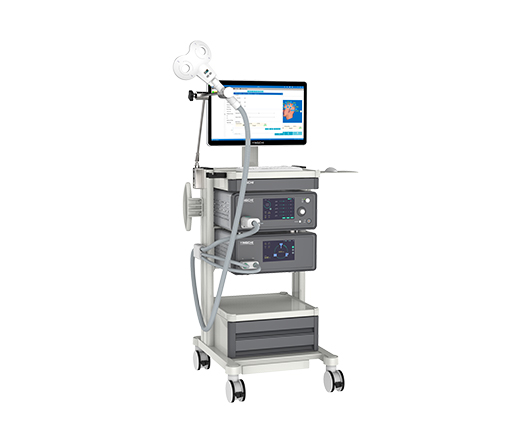
TMS E Series Released
2022.1
YINGCHI TMS has been sold to many countries in Europe/Asia/Africa/South America
Now
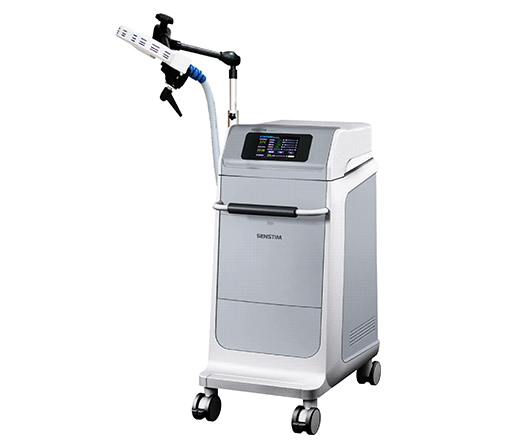
Obtained the license of medical device manufacturer Developed the M-x Pro pulsed magnetic field stimulator prototype
2013.7

China High-tech Enterprise Certificate Shenzhen High-tech Enterprise Certificate
2015.11
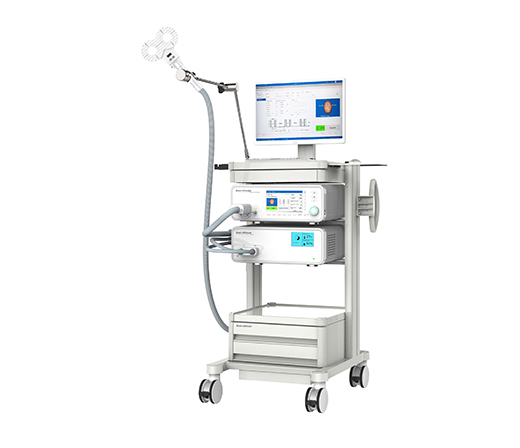
Completed the design and development of the M-x Ultimate TMS prototype
2017.8

M-x Ultimate TMS is TGA approved
2019.5

The development of TMS E series is completed
2021.7
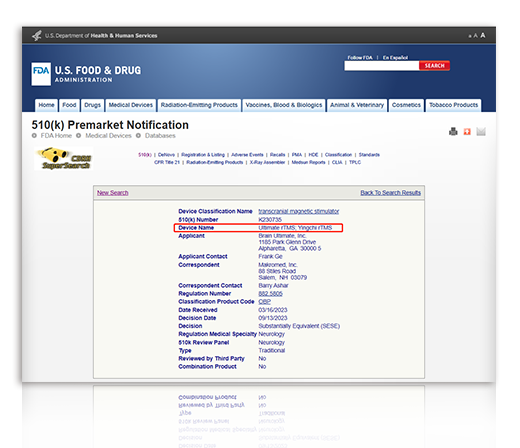
YINGCHI TMS Therapy is FDA cleared for "Treatment of Major Depressive Disorder"
2023.9