Release time :2023-03-15
Source:support@yingchitech.com
Scan:2122
The use of devices to modulate the functioning of neural circuits in the brain is emerging as a promising new approach in the study and treatment of psychiatric disorders. Whether they are surgically implanted in the brain or noninvasively applied to the scalp, devices for modulating circuit function have advanced from the preclinical and first-in-human testing stages to becoming Food and Drug Administration (FDA)-approved treatments such as transcranial magnetic stimulation (TMS) and vagus nerve stimulation (VNS).
Devices modulate circuit function through the interaction of the applied electromagnetic fields and neuronal activity. How these fields interact with the brain can be examined at multiple spatial and temporal scales. Spatially, changes can be observed at the synaptic level; at the level of individual neurons; in local networks of neurons; and at the macro, systems-level brain network scale. Temporally, acute effects occur in the millisecond to second range, and changes can be seen over minutes to hours and over weeks to months. The latter type of long-lasting change is of particular interest in the therapeutic context.[1]
One of the earliest effects found with brain stimulation was its ability to up- and downregulate cortical excitability, with such changes lasting minutes to hours beyond the end of a period of stimulation, typically applied for 10–20 min. Excitability changes have traditionally been measured as changes pre-/post-stimulation intervention in the amplitude of electromyographic potentials due to contractions in peripheral muscles evoked by the stimulation of the motor cortex—motor-evoked potentials. Other ways to measure these changes are with scalp EEG and performance on behavioral tasks[2][3]. A more recently developed method is to observe changes in blood-oxygen-level-dependent fMRI response and resting-state connectivity using MRI [4].
tDCS was shown to bias modulation of LTP in hippocampal slices.[5][6]For more than 30 years from animal experiments and clinical applications, TMS has reached a consensus that high frequency stimulation can cause long-term potentiation (LTP) like increase in nerve excitability in cortex, and low frequency stimulation can cause long-term inhibition (LTD) like decrease in excitability.In rats, TMS has also been shown to affect expression of LTP in rats[7] and expression of genes related to LTP induction [8].
In terms of modulating local circuit dynamics, recent work has suggested that understanding and manipulating endogenous brain oscillations with external stimulation may be a key to controlling and optimizing its effect. Technologies such as transcranial magnetic stimulation (TMS) and transcranial alternating current stimulation (tACS) have shown promise in their ability to drive these oscillations[9][10] .
Through the combined use with PET, MRI and electroencephalogram (EEG), it is found thattranscranial magnetic stimulation (TMS) not only has the stimulation effect on the local cortex, but also has a remote effect through the neural network connection of the stimulation area, causing changes in neurotransmitters, hormones, brain-derived neurotrophic factors, blood flow and metabolism, changes in the basic activity frequency and resonance frequency of brain waves, and modulating brain function through a variety of mechanisms.
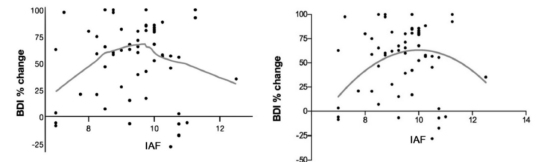
In 2019,a UCLA study showed that when treated with transcranial magnetic stimulation (TMS) at a stimulation frequency of 10 Hz, depressive symptoms improved more if the patient's alpha peak frequency (IAF) value was closer to 10 Hz, suggesting that setting the transcranial magnetic stimulation (TMS) stimulation frequency based on individual neural oscillations may improve depressive symptoms better relative to using a traditional fixed high frequency paradigm.[11]
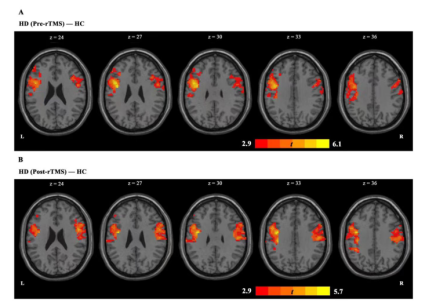
In 2022,The team of Longxiao Wei of Tangdu Hospital of the Air Force Military Medical University uses the transcranial magnetic stimulation (TMS) device for sale (M-100 Ultimate, YINGCHI, Shen Zhen, China) to analyze the functional connections between the default mode network (DMN) and other brain networks of 30 heroin addicts before and after 7 times of transcranial magnetic stimulation. Studies have shown that repetitive transcranial magnetic stimulation can regulate the functional connection between the default functional network and the executive control network, and the change of functional connection between the left inferior parietal lobule and the left middle frontal gyrus may play an important role in reducing drug cue-induced craving.[12]
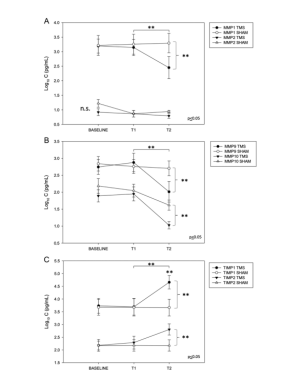
In February 2023, Giovanni Cirillo and others from the University of Campania, Italy, published an article to study the long-term neuromodulatory effect of repetitive transcranial magnetic stimulation (rTMS) on plasma matrix metalloproteinases (MMPs) levels and visuospatial ability in patients with mild cognitive impairment (MCI). The results showed that repeated transcranial magnetic stimulation of bilateral DLPFC for 4 weeks could significantly regulate the plasma levels of MMPs and TIMPs in patients with MCI for a long time (6 months, 24 weeks later). Stimulating DLPFC bytranscranial magnetic stimulation (TMS) to improve the cognitive function of patients with MCI/AD may play a role by inhibiting the expression of MMP-related protein and regulating synaptic remodeling.[13]
[1] Deng Z D, Luber B, Balderston N L, et al. Device-based modulation of neurocircuits as a therapeutic for psychiatric disorders[J]. Annual review of pharmacology and toxicology, 2020, 60: 591-614.
[2] Thut G, Pascual-Leone A. 2010. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 22:219–32 [PubMed: 19862614]
[3] Hoogendam JM, Ramakers GM, Di Lazzaro V. 2010. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3:95–118 [PubMed: 20633438]
[4] Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, et al. 2009. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2:58–80 [PubMed: 20633405]
[5]Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. 2017. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. 10:51–58 [PubMed: 28104085]
[6]Lafon B, Rahman A, Bikson M, Parra LC. 2018. Direct current stimulation alters neuronal input/ output function. Brain Stimul. 10:36–45
[7]Ogiue-Ikeda M, Kawato S, Ueno S. 2003. The effect of repetitive transcranial magnetic stimulation on long-term potentiation in rat hippocampus depends on stimulus intensity. Brain Res. 993:222– 26 [PubMed: 14642850]
[8]Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. 2008. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp. Brain Res 188:249–61 [PubMed: 18385988]
[9]Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. 2011. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol 21:1176–85 [PubMed: 21723129]
[10] Thut G, Bergmann TO, Fröhlich F, Soekadar SR, Brittain JS, et al. 2017. Guiding transcranial brain stimulation by EEG/MEG to interact with ongoing brain activity and associated functions: a position paper. Clin. Neurophysiol 128:843–57 [PubMed: 28233641]
[11]Roelofs CL, Krepel N, Corlier J, et al. Individual alpha frequency proximity associated with repetitive transcranial magnetic stimulation outcome: An independent replication study from the ICON-DB consortium. Clin Neurophysiol. 2021;132(2):643-649.
[12]Jin, Long., Yuan, Menghui., Zhang, Wei., Wang, Lei., Chen, Jiajie.. Default mode network mechanisms of repeated transcranial magnetic stimulation in heroin addiction. Brain imaging and behavior, 2022, .
[13]Cirillo G, Pepe R, Siciliano M, et al. Long-Term Neuromodulatory Effects of Repetitive Transcranial Magnetic Stimulation (rTMS) on Plasmatic Matrix Metalloproteinases (MMPs) Levels and Visuospatial Abilities in Mild Cognitive Impairment (MCI). Int J Mol Sci. 2023;24(4):3231. Published 2023 Feb 6. doi:10.3390/ijms24043231 IF=6.208