Release time :2024-08-07
Source:support@yingchitech.com
Scan:995
Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd.
Swallowing disorders are common complications in stroke patients, with an incidence rate of about 37%-78%. Complications caused by swallowing disorders, such as choking on water, dehydration, electrolyte imbalances, malnutrition, and aspiration pneumonia, severely reduce patients' quality of life and increase their mortality rate. Transcranial Magnetic Stimulation (TMS) is a non-invasive neuromodulation technology that has the potential to regulate neural excitability, promote neuroplasticity, and repair damaged neural functions. Its application in the rehabilitation treatment of neurological, psychiatric, and psychological disorders is increasingly widespread. As research progresses, the therapeutic effects of TMS on post-stroke dysphagia are becoming more prominent, and the clinical demand for TMS treatment among post-stroke dysphagia patients is rapidly increasing.
However, there is currently no unified standard for TMS treatment of post-stroke dysphagia. Clinical staff often set TMS treatment plans for post-stroke dysphagia based on TMS treatment experiences for other diseases or dysfunctions (such as post-stroke hemiplegia) or directly adopt TMS treatment protocols from existing research reports without considering the varying conditions of different patients.
To promote the standardization of TMS treatment for post-stroke dysphagia, improve the efficacy of TMS for post-stroke dysphagia, and reduce the incidence of adverse reactions to TMS treatment, the Chinese Rehabilitation Medical Association released and implemented the TMS treatment standard for post-stroke dysphagia on November 28, 2023.
The main contents of this standard are as follows:

| Indications | Swallowing disorders caused by ischemic stroke, including cerebral thrombosis, cerebral embolism, and lacunar infarction, in patients whose vital signs have stabilized. b. Swallowing disorders caused by hemorrhagic stroke, including hemorrhage in the cerebrum, brainstem, cerebellum, and other regions, in patients where the hemorrhage has been mostly absorbed. |
| Absolute Contraindications | Presence of metallic foreign bodies within 30 centimeters of the treatment area, such as cochlear implants, internal pulse generators, aneurysm clips, and stents. Intracranial hypertension or intracranial infection. c. Severe cardiovascular diseases, especially in patients with pacemakers or cardiac stents. |
| Relative Contraindications | History of epilepsy or electroencephalogram (EEG) indicating epileptiform changes, prohibiting the use of high-frequency and high-intensity stimulation. Severe cerebral hemorrhage, traumatic brain injury, tumors, or infections that may trigger epilepsy. Acute large-area cerebral infarction or multiple intracranial aneurysms. Severe or recent heart attack. Current use of medications that may lower the seizure threshold. Previous or concurrent use of electroconvulsive therapy or vagus nerve stimulation. Sleep deprivation or alcohol dependence. Glaucoma or retinal detachment. Pregnancy. j. Children (during a cold or fever, treatment should not be performed). |
(1) Qualifications for Operators: Physicians and therapists with relevant professional qualifications who have completed specialized transcranial magnetic stimulation training and obtained a training certificate. Therapists must operate under the supervision of a physician.
(2) Equipment Standards: The transcranial magnetic stimulation equipment must comply with national standards.
(3) Treatment Room Requirements:
- The treatment room should be at least 20 square meters in size.
- The room should be equipped with a resuscitation cart, common emergency equipment, and medications.
- Essential emergency equipment includes: cardiopulmonary resuscitation (CPR) equipment, a pulse oximeter, a suction machine, and intravenous infusion equipment.
(4) Treatment Area Setup:
- Each TMS device should be accompanied by a wooden treatment bed or chair.
- No other medical electronic equipment should be placed or used within 2 meters of the TMS device.
(5) Metal and Electronic Device Restrictions:
- Within 30 cm of the coil, there should be no metal objects (excluding those in the oral cavity) or electronic devices. This includes:
- Metal foreign bodies such as cochlear implants, internal pulse generators, aneurysm clips, and stents.
- Bullet fragments and metal brackets.
- Electrical and magnetic items such as mobile phones, credit cards, and calculators.
- Various metal jewelry, watches, and glasses.
(6) Safety Measures:
- All personnel in the treatment room must wear earplugs.
- Pregnant individuals should maintain a distance of at least 0.7 meters from the coil.
(1) Intensity
- For conventional rTMS, the intensity should be set between 80%-120% of the resting motor threshold (RMT).
- For theta burst stimulation (TBS), the intensity should be set at 80% of the active motor threshold (AMT).
(2) Frequency
- Low frequency: typically 1Hz.
- High frequency: commonly 5Hz or 10Hz, with earlier studies also using 3Hz.
(3) Standardized Settings for Burst Duration and Inter-burst Interval
- Refer to the TMS safety guidelines established by the International Society of Transcranial Stimulation in 2009. When the intensity and frequency are fixed, the maximum duration for rTMS should not exceed the range listed in the lower part of the table, and the minimum interval should not be shorter than the range listed in the upper part of the table.
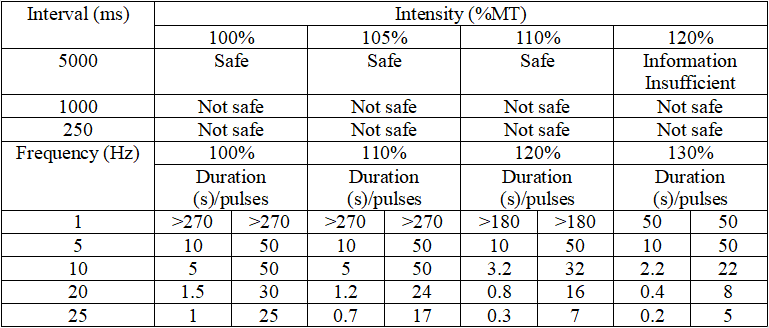
(4) Treatment Duration and Total Number of Pulses
- For rTMS, the treatment duration should be 10-30 minutes, with the total number of pulses set between 600-1200.
- For continuous theta burst stimulation (cTBS), the treatment duration should be 40 seconds.
- For intermittent theta burst stimulation (iTBS), the treatment duration should be 3 minutes and 20 seconds, with the total number of pulses set at 600.
(5) Course of Treatment
- Based on current clinical and research experience, a single course of treatment for post-stroke dysphagia should be 1-3 weeks.
- Depending on the patient's condition, one or more treatment courses can be administered.
(1) Stages of Post-Stroke Dysphagia
- Acute Phase: Duration < 2 weeks
- Recovery Phase: 2 weeks ≤ Duration ≤ 6 months
- Chronic Phase: Duration > 6 months
(2) Treatment Plans for Cerebral Hemisphere Stroke Patients
Protocol A: High-Frequency rTMS on the Affected Side
- Effective for patients in the acute phase.
- Effects are not significant for patients in the recovery and chronic phases.
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| Affected Pharyngeal Motor Cortex | 3Hz | 120%RMT | 300 | 5 days |
| Affected Superior Laryngeal Motor Cortex | 3Hz | 90%RMT | 1200 | 5 days |
| Affected Superior Laryngeal Motor Cortex | 10Hz | 110%RMT | 900 | 10 days |
| Unaffected Pharyngeal Motor Cortex | 5Hz | 90%RMT | 500 | 14 days |
Protocol B: High-Frequency rTMS on the Unaffected Side
- Effective for patients in the recovery phase.
- Significant reduction in aspiration-leak scores compared to before treatment, especially improvements in aspiration and residue, though swallowing delay and abnormal pharyngeal transit time show no significant changes.
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| Unaffected Pharyngeal Motor Cortex | 5Hz | 90%RMT | 500 | 14 days |
Protocol C: Low-Frequency rTMS on the Unaffected Side
- Effective for patients in the acute, recovery, and chronic phases.
Acute Phase
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| Unaffected Superior Laryngeal Motor Cortex | 1Hz | 100%RMT | 1200 | 5 days |
Recovery Phase
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| Unaffected Pharyngeal Motor Cortex | 1Hz | 100%RMT | 1200 | 10 days |
| Unaffected Superior Laryngeal Motor Cortex | 1Hz | 120%RMT | 1200 | 5 days |
Chronic Phase
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| Unaffected Mylohyoid Motor Cortex | 1Hz | 120%RMT | 1200 | 5 days |
Protocol D: Bilateral High-Frequency rTMS
- Effective for patients in the recovery phase.
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| Bilateral Suprahyoid Motor Cortex | 10Hz | 90%RMT | 500 | 14 days |
Protocol E: High-Frequency rTMS on the Cerebellum
- Effective for patients in the recovery phase.
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| The Pharyngeal Motor Area of the Cerebellum Ipsilateral to the Unaffected Cerebral Hemisphere | 10Hz | 110%RMT | 1200 | 18 days |
(3) Treatment Plans for Brainstem Stroke Patients
- High-Frequency rTMS on the Affected Side or Bilateral High-Frequency rTMS
- Effective for patients in the recovery phase.
- Bilateral high-frequency rTMS is superior to unilateral high-frequency rTMS and provides effects that can last up to 2 months. Therefore, for brainstem stroke patients, bilateral high-frequency rTMS should be preferred when conditions permit.
| Target | Frequency | Intensity | Total Pulses | Course of Treatment |
| Bilateral Esophageal Motor Cortex | 3Hz | 130%RMT | 300 | 5 days |
Headache
Facial or limb twitching
Temporary hearing loss
Tinnitus
Seizures
Dizziness
Mood changes
(1) In case of seizures, loss of consciousness, fainting, or other emergencies during treatment:
a. Immediately stop the assessment.
b. Ensure the patient’s airway is clear.
c. Closely monitor the patient’s vital signs.
d. For cardiac arrest or respiratory cessation, immediately perform cardiopulmonary resuscitation (CPR).
e. Assess the patient’s consciousness level.
f. Contact the patient’s family to inform them of the patient’s condition and the measures taken.
(2) In case of a seizure during treatment:
a. Place the patient in a lying position on the bed or on a flat surface nearby.
b. Maintain airway patency.
c. Remove secretions from the mouth.
d. Unfasten the collar and belt, support the patient’s head to prevent neck hyperextension and maintain airway patency.
e. Use a tongue depressor or dental pad to prevent biting of the tongue or cheeks. During the clonic phase, limit movements if necessary, but do not apply force to avoid fractures or dislocations.
f. Administer low-flow oxygen.
g. Contact a neurologist immediately for assistance.
h. Sedation: Establish an intravenous line and administer anticonvulsant medication rapidly and in adequate amounts to control seizures. Administer diazepam 10-20 mg slowly intravenously.
i. Transfer to a neurology department for further treatment if necessary.
(3) If adverse reactions such as headache, facial or limb twitching, temporary hearing loss, tinnitus, dizziness, or mood changes occur during treatment:
Immediately stop TMS treatment.
Once symptoms alleviate, if symptoms persist, seek further evaluation and treatment from relevant specialists.

This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.