Release time :2023-01-04
Source:support@yingchitech.com
Scan:1843
Transcranial magnetic stimulation (TMS) is a non-invasive neuromodulation technology. The time-varying pulsed magnetic field can penetrate the skull non-invasively, act on the central nervous system, generate induced currents, and cause a series of physiological and biochemical reactions, thus affecting brain metabolism and neuronal excitability, so as to improve and treat mental and neurological diseases.
TMS mainly achieves the function of excitation or inhibition of the local cerebral cortex by changing the stimulation frequency, and treats diseases by bidirectionally regulating the balance between the excitation and inhibition functions of the brain.
In addition to ongoing clinical trials, FDA approval of indications related to transcranial magnetic stimulation therapy also sets the stage for clinical use. Here we review indications approved by the FDA for TMS and recommended indications in rTMS Clinical Treatment Guidelinesof International Ferderation of Clinical Neurophysiology (IFCN).
In 2008, FDA approved TMS for the treatment of major depression;
In 2013, FDA approved TMS for the treatment of migraine;
In 2020, FDA approved TMS for the treatment of obsessive-compulsive disorder (OCD);
In 2021, FDA approved TMS for the treatment of depression with anxiety symptoms.
Table 1 Guidelines of TMS Therapy
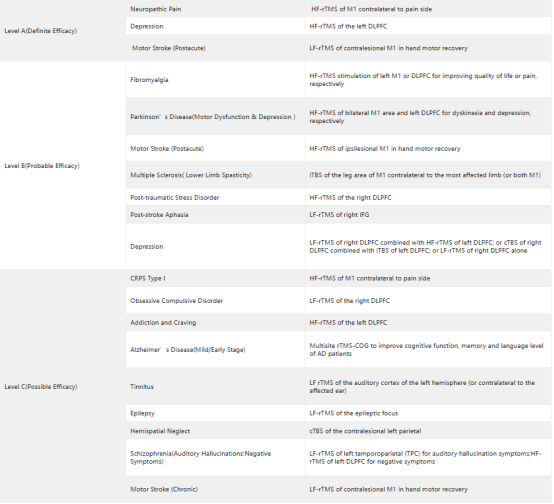
It has been more than a decade since the US Food and Drug Administration (FDA) first approved transcranial magnetic stimulation (TMS). The improvement and optimization of TMS therapy paves the way for the improvement and broadening of emerging techniques for clinical application of TMS, such as electromagnetic coil techniques, pulse training regimens (such as 18/20 Hz stimulation and iTBS), and neural navigation system techniques, among others[2].
High frequency stimulation of the left dorsolateral prefrontal lobe (DLPFC) using A figure-of-eight coil has been approved by the FDA and Class A guidelines are recommended. However, some literature reviews have shown that stimulation of bilateral dorsalateral prefrontal lobes (DMPFC) with biconical coils can also be effective in treating depression [3], and controlled trials have shown that stimulation of bilateral dorsalateral anterior cingulate (dACC) with biconical coils is more effective in reducing depression scores [4]. In 2020, the FDA approved biconical coils for OCD. Stimulation of orbitofrontal cortex, DMPFC and ACC with biconical coils can effectively treat OCD.
YINGCHI TMS has utilized the double cone coil, which is capable of accessing deeper cortical tissue, in order to excite the pelvic floor, the lower limb motor representation, and the anterior cingulate cortex (ACC). All three of these areas were stimulated by the double cone coil (anterior cingulate cortex). It is helpful in the treatment of the major depressive disorder, obsessive-compulsive disorder, stroke, and other conditions, and it has the capacity to stimulate reactions in the lower extremities.

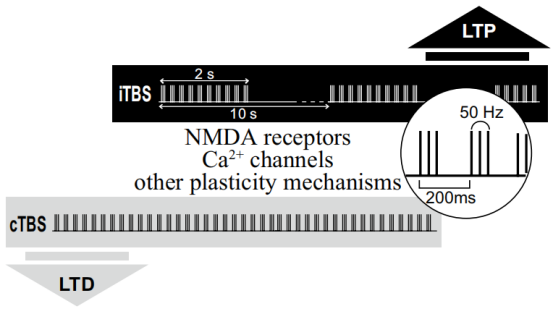
New pulse parameters, such as theta burst stimulation have also demonstrated utility in improving TMS therapeutic effects. In 2018, the FDA approved the first treatment system to receive an intermittent Theta Burst stimulation (iTBS) protocol, with Theta Burst stimulation, to treat MDD in adults whose symptoms were previously unable to improve with antidepressants [2].
Yingchi TMS can provide theta-burst stimulation (TBS) without intensity decay. The E Series also makes it possible to customize TBS, which will be a useful tool for professionals to explore new possibilities in their research.
Traditional positioning methods are unable to solve the problem of accurate, intelligent and efficient accurate positioning and repeatability. In 2020, the FDA approved an MRI Neuronavigational system based on neuroimaging for precise positioning of therapeutic coils.
YINGCHI TMS 3D navigation system can import individual structure and functional MRI data to realize individualized accurate positioning based on the structure and functional loop, and truly solve the problem of individual differences. It also has the function of target memory, which can realize accurate and repeated positioning. In the treatment, the target can also be monitored in real time, and once the target is offset, it will automatically prompt to ensure the accuracy and consistency of the target positioning for the whole course of treatment, so as to improve the treatment efficiency and effect, and also provide a technical basis for high-end scientific research.
In 2021 year, an accelerated, high-dose, modulated, functionally connected MRI (fcMRI) guided TMS therapy protocol, Stanford Neuromodulation Therapy, was developed to optimize the treatment of drug-resistant depression using the stimulus parameters provided by neuroscience [5]. SNT includes 1) an efficient form of rTMS, termed intermittent theta-burst stimulation(iTBS) ; 2) treatment with multiple iTBS sessions per day at optimally spaced intervals ; 3) application of
a higher overall pulse dose of stimulation; and 4) personalized targeting for application of the stimulation of the left DLPFC to subgenual anterior cingulate cortex (sgACC) circuit. The short treatment course and the high antidepressant efficacy of SNT present an opportunity to treat patients in emergency or inpatient settings where rapid-acting treatments are needed.In 2022, the SAINT Neuromodulation System received FDA approval for the non-invasive, individualized and precise treatment of major depression.
Yingzhi TMS iTBS model combined with 3D navigation system precise positioning helps accelerate the realization of therapy, bringing new hope for the treatment of major depression.
The TMS therapy field has grown substantially over the past two decades, moving from promising research findings to numerous FDA-approved indication and new technology with broad ranging treatment utility in neurological and psychiatric disorders. In this TMS therapy review, we reviewed some important milestones of TMS therapy. For additional information in the field of TMS therapy or related TMS devices, you can also visit www.yingchi-tms.com!
[1]*Lefaucheur, J. P. , Aleman, A. , Baeken, C. , Benninger, D. H. , & Ziemann, U. . (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rtms): an update (2014–2018). Clinical Neurophysiology, 131(2)
[2]Cohen, Samantha L., Bikson, Marom., Badran, Bashar W., and George, Mark S.. "A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices." Brain stimulation 15.1(2021):73-75.
[3] Monteiro, D.C., & Cantilino, A..(2018).Use of a double〤one coil in transcranial magnetic stimulation for depression treatment.Neuromodulation.
[4] Kreuzer, P.M., Schecklmann, M., Lehner, A., Wetter, T.C., & Langguth, B..(2014).The acdc pilot trial: targeting the anterior cingulate by double cone coil rtms for the treatment of depression.Brain Stimulation, 8(2), 240-246.
[5]Cole, Eleanor J., Phillips, Angela L., Bentzley, Brandon S., Stimpson, Katy H., and Nejad, Romina.. "Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial." The American journal of psychiatry 179.2(2021):132-141.