Release time :2025-03-18
Source:support@yingchitech.com
Scan:111

According to surveys, 7%-12% of pregnant women worldwide are affected by major depressive disorder (MDD). If depression during pregnancy is not treated, it can lead to numerous adverse consequences. Current primary treatment methods include psychotherapy, medication, and electroconvulsive therapy, but these come with side effects and risks. In recent years, transcranial magnetic stimulation (TMS), as a non-pharmacological treatment option, has provided a new choice for depression treatment. TMS offers advantages such as high safety and fewer adverse reactions, bringing hope to pregnant women with depression.
Pregnant women with depression are a special population, and the primary consideration during treatment is the impact of TMS (Transcranial Magnetic Stimulation) on the fetus. The magnetic field generated by the stimulation coil decreases exponentially with distance, and generally, the magnetic field stimulating the head does not directly affect the fetus. Existing literature has reported successful treatment of depression in pregnant women using TMS with no adverse effects on the fetus. It is not recommended to stimulate the lumbar spine or abdomen of pregnant women. The 2009 TMS safety guidelines state that long-term rTMS operators and pregnant women should maintain a distance of at least 70 cm from the coil.
The latest published review, Transcranial Magnetic Stimulation in Pregnancy: Efficacy, Safety, and Future Implications for Perinatal Mental Health Care, conducts a qualitative assessment of existing literature, focusing on the safety and efficacy of TMS as a treatment method for depression during pregnancy.

This study strictly adheres to the guidelines of the PRISMA statement.
EMBASE, MedLINE, PsychINFO, and CINAHL databases.
Randomized controlled trials (RCTs) and open-label trials were included, with a focus on TMS as a treatment intervention for depression during pregnancy.
Study participants were pregnant women at any stage of pregnancy. Participants were individuals diagnosed with unipolar major depressive disorder. There were no restrictions on the use of pharmacotherapy before, during, or after TMS treatment.
Studies focused on the perinatal period rather than the prenatal stage. Studies using TMS to treat conditions unrelated to unipolar major depressive disorder (e.g., migraines). Studies using TMS for maintenance therapy during pregnancy rather than for the treatment of acute major depressive episodes. Studies not published in English.
After screening and careful evaluation, only three studies met the strict inclusion criteria, including two open-label studies and one RCT.
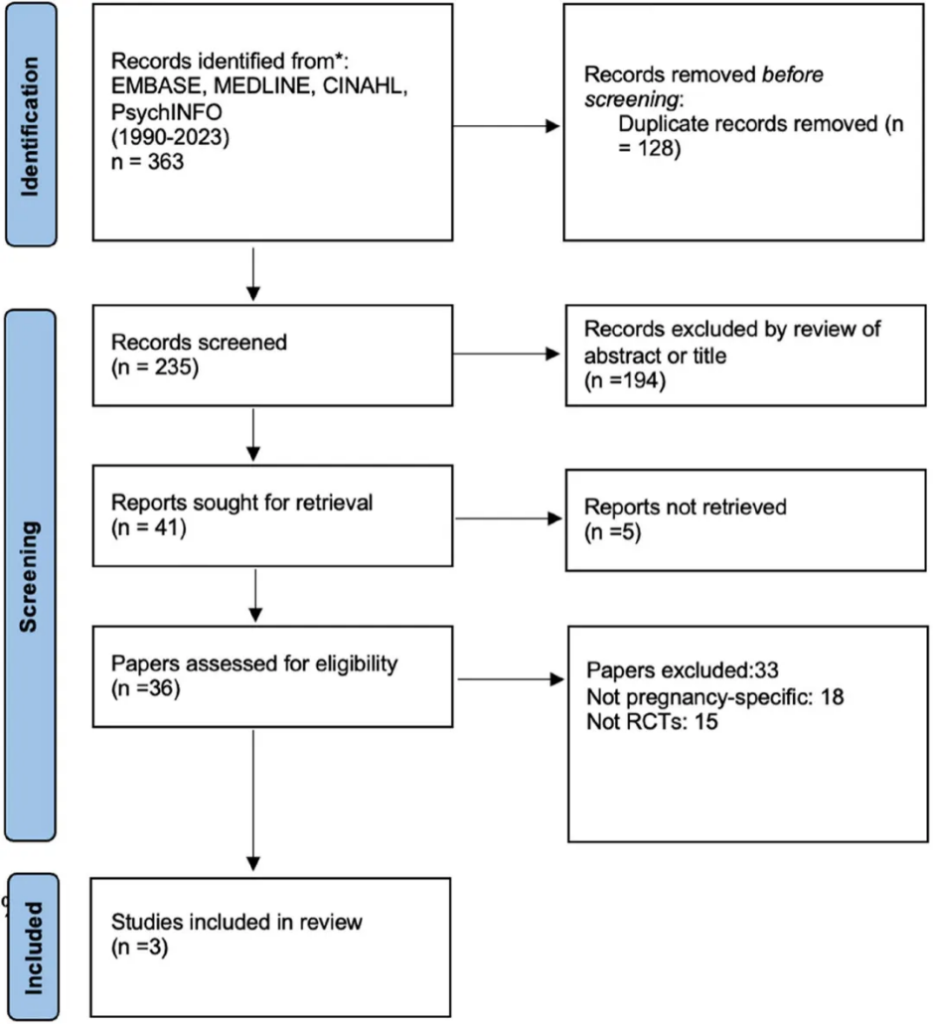
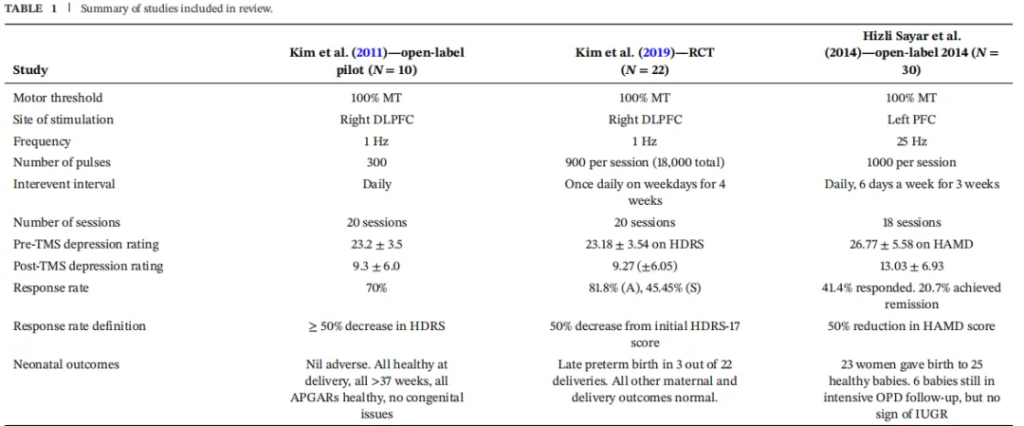
Kim et al. (2011) Open-Label Study:
Study Participants: 10 pregnant women in the second or third trimester (gestational age 14-34 weeks) with major depressive disorder.
Intervention: Low-frequency 1 Hz stimulation to the right dorsolateral prefrontal cortex, administered 5 times per week for a total of 20 sessions.
Results: 70% of patients responded to the treatment (depression scale score reduction ≥50%), and 30% achieved remission (depression scale score <8). All infants were born healthy with no adverse outcomes.
Kim et al. (2019) Randomized Controlled Trial :
Study Participants: 22 pregnant women in the second or third trimester (gestational age 14-34 weeks) with major depressive disorder, randomly assigned to either a real or sham TMS group.
Intervention: Low-frequency 1 Hz stimulation to the right dorsolateral prefrontal cortex, administered 5 times per week for a total of 20 sessions.
Results: In the real stimulation group, 81.8% of patients responded to the treatment, and 27.3% achieved remission; in the sham stimulation group, 45.5% responded, and 18.8% achieved remission. All infants were born healthy with no adverse outcomes. However, it is worth noting that due to the small sample size, neither the response rates nor remission rates reached statistical significance.
Hizli et al. (2014) Open-Label Study:
Study Participants: 30 pregnant women with major depressive disorder (gestational age 5-32 weeks).
Intervention: High-frequency 25 Hz stimulation to the left dorsolateral prefrontal cortex, administered 6 times per week for a total of 18 sessions.
Results: 41.4% of patients responded to the treatment, and 20.7% achieved remission. All infants were born healthy with no adverse outcomes.
All studies reported good tolerability among patients. The most common side effect was mild headache, with other transient side effects including dizziness and nausea, which resolved spontaneously after treatment. No seizures were reported.
The Potential of TMS in Treating Depression During Pregnancy
The review suggests that TMS targeting the dorsolateral prefrontal cortex is safe and effective for pregnant women with major depressive disorder, significantly reducing depression scale scores and improving depressive symptoms, with good tolerability for both mother and infant. However, the existing studies have small sample sizes, a high risk of bias, and only one randomized controlled trial (with 22 participants), which falls far short of an ideal sample size. Overall, the current evidence indicates that transcranial magnetic stimulation holds promise as a valuable treatment option for pregnant women with depression.
01. There is a need for larger-scale, more rigorous randomized controlled trials to clearly define the applicable population for TMS in depression.
02. Exploration of diverse TMS protocols (e.g., theta-burst stimulation) is warranted.
03. Long-term longitudinal studies are needed to evaluate the potential impact of TMS on the developmental outcomes of offspring of pregnant women with depression, in order to develop evidence-based guidelines and optimize mental health care for pregnant women during pregnancy.

1. This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.
2. Reference:
Angeline, S., Tiyatiye, B., & Akosile, W. (2025). Transcranial Magnetic Stimulation in Pregnancy: Efficacy, Safety, and Future Implications for Perinatal Mental Health Care. Brain and behavior, 15(2), e70304. https://doi.org/10.1002/brb3.70304