Release time :2022-07-08
Source:support@yingchitech.com
Scan:2245
Since the 1980s, Transcranial Magnetic Stimulation (TMS) has been used to study the nerve fibers that carry information about movements from the brain to the spinal cord and on to the muscles. In 2004, Chinese Taiwanese scholar Ying-Ju Huang first proposed the theta burst stimulation (TBS) model and proved that it is safe and can target specific populations of neurons in the motor cortex.[1] Subsequently, the clinical use of TBS has become increasingly valuable and plays an important role in the treatment of related diseases.
Through all these research over many years, the fnal TBS protocol of rTMS in humans involved the application of three bursts of 50 Hz stimulation, delivered at an interburst interval frequency of 5 Hz and an intensity of 80% AMT. When applied continuously, for a period of 20 or 40 s (300 or 600 pulses), known as ‘continuous’ theta burst stimulation or ‘cTBS’, this led to an LTD-like reduction in MEP size and corticospinal activity lasting for up 20 or 60 min, respectively, after stimulation. Conversely, an intermittent pattern of stimulation (known as ‘intermittent’ theta burst stimulation or ‘iTBS’), in which 600 pulses are delivered in 2 s trains of theta burst stimulation repeated every 10 s for 20 cycles (a total of 190 s), led to signifcant LTP-like increases in MEP size and excitability for up to 20 min.[2][3]
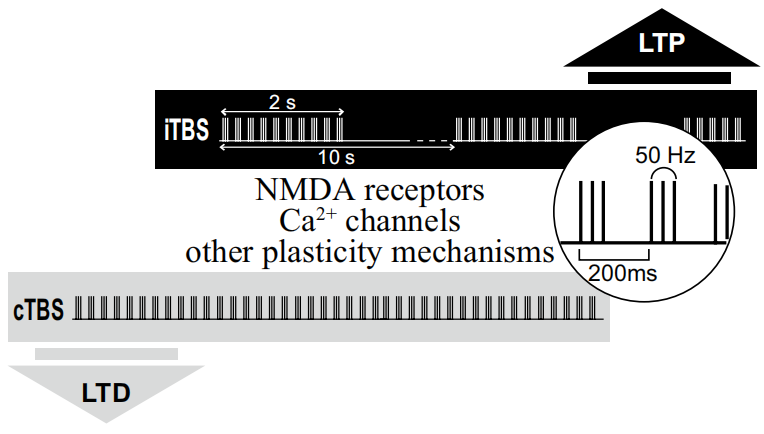
Initial work involved testing of intracortical inhibition and excitability circuits and H refex. The low intensity of stimulation, presence of effects of stimulation at short latency, as well as demonstration of GABAa-induced intracortical inhibition effects, with the absence of any effects of TBS on H refexes, demonstrated that TBS effects were cortical.
An original model to explain the bidirectional effects of theta burst stimulation was developed based on the mechanisms of post-synaptic plasticity described at a cellular level, which has been supported by pharmacological studies using NMDA antagonists and calcium blockers.
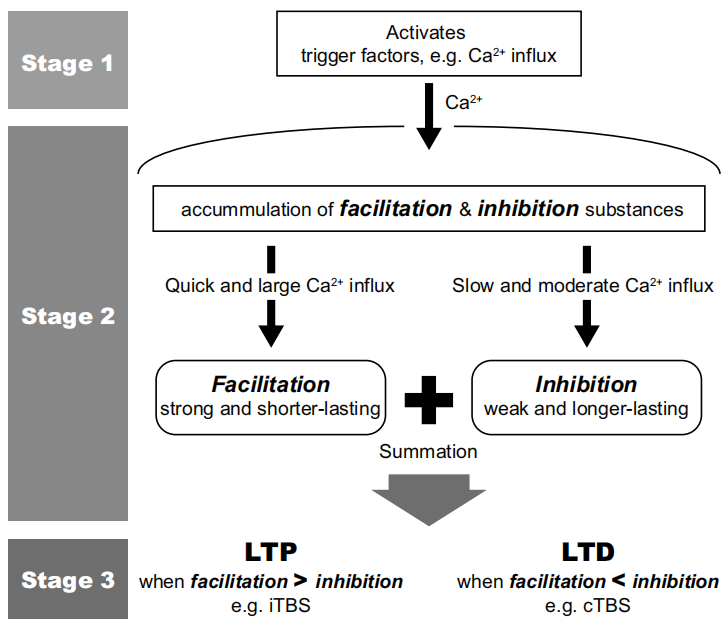
However, more recent data suggest that, other than the glutaminergic pathway described in the theoretic model, GABAergic circuits are also involved. Magnetic resonance spectroscopy demonstrated that GABA, instead of glutamine, was signifcantly changed by cTBS. Moreover, GABA release is known to be involved in iTBS-induced potentiation and the effects of TBS could be modulated by the blockage of GABAergic receptors.On the other hand, TBS has been found to induce long-lasting changes in GABAergic interneurons in animal studies. Hence, newer mechanisms of action of TBS will need to be investigated in light of these recent fndings.
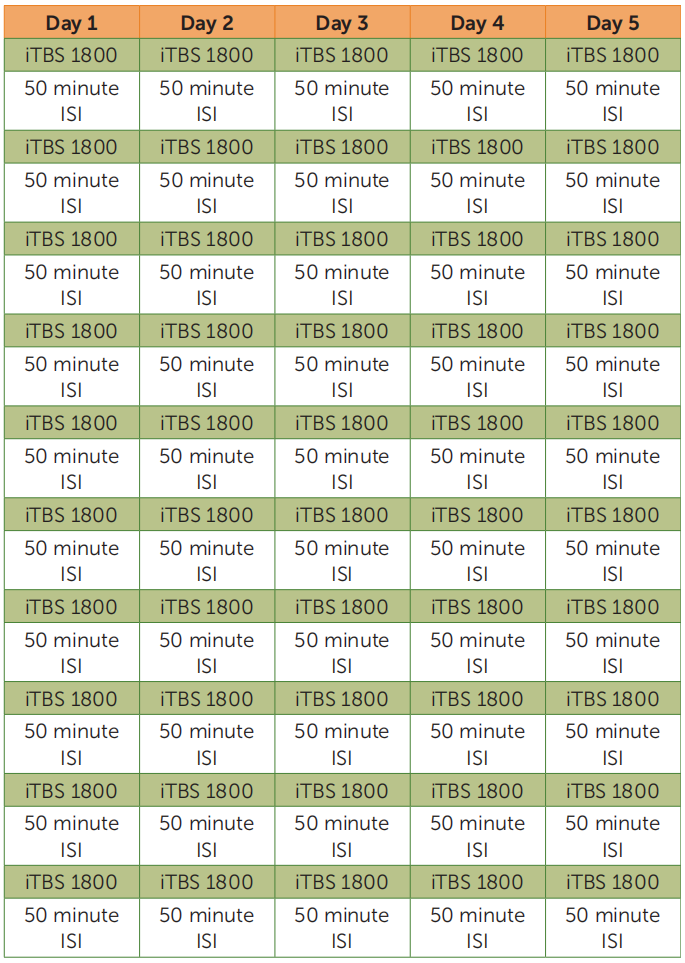
Depression is the leading cause of disability worldwide, and half of patients with depression have treatment-resistant depression. Intermittent theta-burst stimulation (iTBS) is approved by the U.S. Food and Drug Administration for the treatment of treatment-resistant depression but is limited by suboptimal efficacy and a 6-week duration. The Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT), a new TBS-based treatment for refractory depression, was recently developed by Stanford University School of Medicine and published in the American Journal of Psychiatry. The protocal is that fifty iTBS sessions (1,800 pulses per session, 50-minute intersession interval) were delivered as 10 daily sessions over 5 consecutive days at 90% resting motor threshold (adjusted for cortical depth).[4] The results of the study found that the Montgomery-Åsberg Depression Rating Scale scores of patients decreased significantly after treatment with no adverse effects. The treatment was found to be significantly effective and safe for the treatment of refractory depression.SNT, a high-dose iTBS protocol with functional-connectivity-guided targeting, was more effective than sham stimulation for treatment-resistant depression.And the team recently updated their findings with a randomized, double-blind controlled trial that also demonstrated the effectiveness of the therapy, and they will next further compare the difference in efficacy of this therapy with different treatments.[5]
In general,theta burst stimulation was developed to enable the rapid induction of long-lasting changes in excitability and function of cortical areas in human participants. This technique has faced challenges of reproducibility also observed in other forms of non-invasive brain stimulation techniques. Solving these will involve a better understanding of the disease processes and stimulation techniques, which will allow a better prediction of after-effects. Nevertheless, TBS remains an efficient and safe technique providing the opportunity to alter human brain function in health and disease.
【1】Huang YZ, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol. 2004;115(5):1069-1075. doi:10.1016/j.clinph.2003.12.026
【2】Rounis E, Huang YZ. Theta burst stimulation in humans: a need for better understanding effects of brain stimulation in health and disease. Exp Brain Res. 2020;238(7-8):1707-1714. doi:10.1007/s00221-020-05880-1
【3】Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201-206. doi:10.1016/j.neuron.2004.12.033
【4】Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020;177(8):716-726. doi:10.1176/appi.ajp.2019.19070720
【5】Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429